Spinal muscular atrophy (SMA) is a group of neuromuscular disorders that result in the loss of motor neurons and progressive muscle wasting.[1] The severity of symptoms and age of onset varies by the type.[1] Some types are apparent at or before birth while others are not apparent until adulthood.[1] All generally result in worsening muscle weakness associated with muscle twitching.[1][3] Arm, leg and respiratory muscles are generally affected first.[3][4] Associated problems may include problems with swallowing, scoliosis, and joint contractures.[2][4] SMA is a leading genetic cause of death in infants.[5]
Spinal muscular atrophy is due to a genetic defect in the SMN1 gene.[1][2] They are inherited from a person's parents in an autosomal recessive manner.[1] The SMN1 gene encodes SMN, a protein necessary for survival of motor neurons.[4] Loss of these neurons prevents the sending of signals between the brain and skeletal muscles.[4] Diagnosis is suspected based on symptoms and confirmed by genetic testing.[1]
Treatments include supportive care such as physical therapy, nutrition support, and mechanical ventilation.[1] The medication nusinersen, which is injected around the spinal cord, slows the progression of the disease and improves muscle function.[1][3] In 2019, the gene therapy onasemnogene abeparvovec was approved in the US as a treatment for children under 24 months.[5] Outcomes vary by type from a life expectancy of a few months to mild muscle weakness with a normal life expectancy.[4] The condition affects about 1 in 10,000 people at birth.[2]
Signs and symptoms
The symptoms vary depending on the SMA type, the stage of the disease as well as individual factors. Signs and symptoms below are most common in the severe SMA type 0/I:[11][medical citation needed]
- Areflexia, particularly in extremities
- Overall muscle weakness, poor muscle tone, limpness or a tendency to flop
- Difficulty achieving developmental milestones, difficulty sitting/standing/walking
- In small children: adopting of a frog-leg position when sitting (hips abducted and knees flexed)
- Loss of strength of the respiratory muscles: weak cough, weak cry (infants), accumulation of secretions in the lungs or throat, respiratory distress
- Bell-shaped torso (caused by using only abdominal muscles for respiration) in severe SMA type
- Fasciculations (twitching) of the tongue
- Difficulty sucking or swallowing, poor feeding
Causes
Human chromosome 5 contains two nearly identical genes at location 5q13: a telomeric copy SMN1 and a centromeric copy SMN2. In healthy individuals, the SMN1 gene codes the survival of motor neuron protein (SMN) which, as its name says, plays a crucial role in survival of motor neurons. The SMN2 gene, on the other hand – due to a variation in a single nucleotide (840.C→T) – undergoes alternative splicing at the junction of intron 6 to exon 8, with only 10–20% of SMN2 transcripts coding a fully functional survival of motor neuron protein (SMN-fl) and 80–90% of transcripts resulting in a truncated protein compound (SMNΔ7) which is rapidly degraded in the cell.[13]
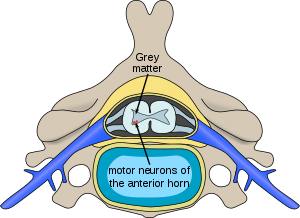
In individuals affected by SMA, the SMN1 gene is mutated in such a way that it is unable to correctly code the SMN protein – due to either a deletion[14] occurring at exon 7[15] or to other point mutations (frequently resulting in the functional conversion of the SMN1 sequence into SMN2). Almost all people, however, have at least one functional copy of the SMN2 gene (with most having 2–4 of them) which still codes small amounts of SMN protein – around 10–20% of the normal level – allowing some neurons to survive. In the long run, however, reduced availability of the SMN protein results in gradual death of motor neuron cells in the anterior horn of spinal cord and the brain. Muscles that depend on these motor neurons for neural input now have decreased innervation (also called denervation), and therefore have decreased input from the central nervous system (CNS). Decreased impulse transmission through the motor neurons leads to decreased contractile activity of the denervated muscle. Consequently, denervated muscles undergo progressive atrophy (waste away).[citation needed]
Muscles of lower extremities are usually affected first, followed by muscles of upper extremities, spine and neck and, in more severe cases, pulmonary and mastication muscles. Proximal muscles are always affected earlier and to a greater degree than distal.[16][citation needed]
The severity of SMA symptoms is broadly related to how well the remaining SMN2 genes can make up for the loss of function of SMN1. This is partly related to the number of SMN2 gene copies present on the chromosome. Whilst healthy individuals carry two SMN2 gene copies, people with SMA can have anything between 1 and 4 (or more) of them, with the greater the number of SMN2 copies, the milder the disease severity. Thus, most SMA type I babies have one or two SMN2 copies; people with SMA II and III usually have at least three SMN2 copies; and people with SMA IV normally have at least four of them. However, the correlation between symptom severity and SMN2 copy number is not absolute, and there seem to exist other factors affecting the disease phenotype.[17]
Spinal muscular atrophy is inherited in an autosomal recessive pattern, which means that the defective gene is located on an autosome. Two copies of the defective gene – one from each parent – are required to inherit the disorder: the parents may be carriers and not personally affected. SMA seems to appear de novo (i.e., without any hereditary causes) in around 2–4% of cases.
Spinal muscular atrophy affects individuals of all ethnic groups, unlike other well known autosomal recessive disorders, such as sickle cell disease and cystic fibrosis, which have significant differences in occurrence rate among ethnic groups. The overall prevalence of SMA, of all types and across all ethnic groups, is in the range of 1 per 10,000 individuals; the gene frequency is around 1:100, therefore, approximately one in 50 persons are carriers.[18][19] There are no known health consequences of being a carrier. A person may learn carrier status only if one's child is affected by SMA or by having the SMN1 gene sequenced.
Affected siblings usually have a very similar form of SMA. However, occurrences of different SMA types among siblings do exist – while rare, these cases might be due to additional de novo deletions of the SMN gene, not involving the NAIP gene, or the differences in SMN2 copy numbers